FDA approves new cholesterol lowering drug
By Debra Goldschmidt, CNN
Updated 2341 GMT (0641 HKT) July 24, 2015
The FDA approves a new cholesterol drug
Story highlights
Praluent is an alternative cholesterol lowering medication for patients who don't respond to statins or can't take them
Praluent is the first in a new class of drugs called PCSK9 inhibitors
The FDA has until the end of August to decide on Repatha, another new PCSK9 inhibitor
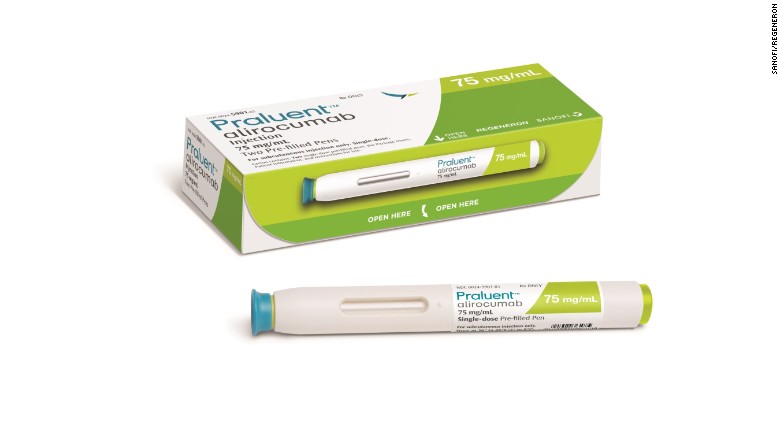
Atlanta (CNN)The FDA approved the new cholesterol lowering drug alirocumab, brand name Praluent, on Friday. The injectable drug, from Regeneron and Sanofi, is the first in a new class of drugs called PCSK9 inhibitors.
The drug works by making the liver more efficient at getting rid of LDL, or bad cholesterol.
Praluent is approved for patients with heterozygous familial hypercholesterolemia, or HeFh, which is an inherited condition that causes high LDL cholesterol levels. It is also approved for patients who have had a heart attack or stroke. "It focuses on those who've truly had clinical disease or those who start out with such high levels of LDL they can't get anywhere near where they should be and I think those are the most at-risk people," said Dr. Donald A. Smith, associate professor of medicine and cardiology at Mount Sinai Hospital in New York.
This offers another treatment option for patients who aren't responding to currently available medications or who can't take them because they experience side effects.
CNN Explains: Cholesterol 01:46
PLAY VIDEO
The currently available medications for lowering cholesterol are statins, of which there are seven on the market.
Smith thinks that with more research, this drug could be approved more broadly for patients at high risk who have not had a heart attack or stroke.
Regeneron said Friday that the drug will be available as soon as early next week in two doses as a prefilled pen that patients administer to themselves once every two weeks. The wholesale cost of the drug will be about $1,200 per month. The cost for patients will depend on their insurance plan. In comparison, statins cost between $500 and $700 a year for name-brand versions and $48 a year for generics.
As with most medications, the drug has some potential side effects, including itching, swelling, pain or bruising from the injection. Patients can also experience cold- and flu-like symptoms.
An FDA advisory committee recommended the drug for approval in June. Friday was the deadline for the agency to make a decision on whether to approve the drug.
In June the same advisory committee also recommended another new drug, evolocumab, brand name Repatha, which is still being considered by the FDA and was approved in Europe earlier this week.
The new drugs are a "powerful new way of lowering the bad form of cholesterol, and that has profound implications in dealing with the burden of vascular disease," which can lead to heart attacks and stroke, Dr. Elliott Antman, president of the American Heart Association, said in June.
CNN's Sandee LaMotte, Carina Storrs and Liza Lucas contributed to this report
Sem comentários:
Enviar um comentário